Image: Fa. Viessmann
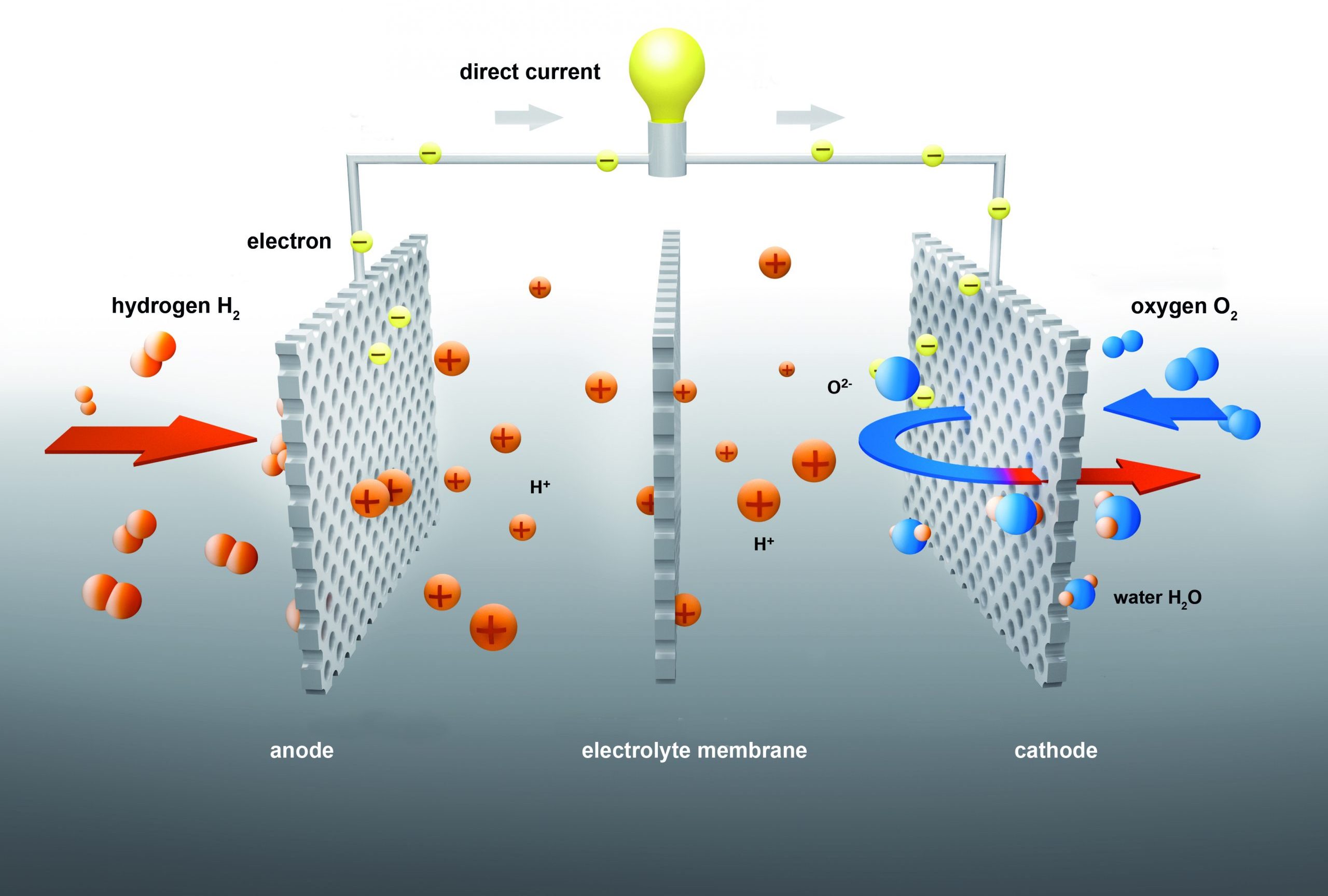
At the anode, hydrogen is oxidized into two protons (H+) and two electrons (e–). The protons migrate through the membrane (or an electrolyte solution). The electrons move through the anode to the cathode and current flows. At the cathode, the electrons reduce the oxygen and in the last step, the oxygen ions combine with the protons to form water.
Today, the focus is on fuel cells that work with a proton exchange membrane (PEMFC) instead of liquid electrolytes. In comparison, these systems are very durable and require little maintenance, contain no alkalis or acids, are quickly ready for operation and work with ambient air instead of pure oxygen. They are already being used in some heaters and as prototypes in cars, buses and vans.
