
Larger particles, better Lithium transport: Researchers around Prof. Bettina Lotsch’s Team at MPI Stuttgart explored how different particle sizes within the solid electrolyte affect its conductivity. (Image: V. Hiendl / e-conversion)
Solid-state batteries are seen as a beacon of hope for future energy storage solutions. They promise higher energy densities, shorter charging times, and increased safety compared to conventional lithium-ion batteries. A key component in solid-state batteries is the electrolyte: instead of a liquid, a solid material is used to transport the charge carriers (lithium ions) between the anode and cathode. However, many challenges remain before these batteries reach market maturity — one of them being the development of high-performance solid electrolytes that can be processed into thin, efficient, and reliable layers.
Among the most promising solid electrolytes are members of the thiophosphate family. In a recent study, the team led by Prof. Bettina V. Lotsch at the Max Planck Institute for Solid-State Research in Stuttgart systematically investigated one such compound: tetragonal Li₇SiPS₈. “This material exhibits high ionic conductivity and favorable mechanical properties, but — unlike established materials — it contains silicon, a much more cost-effective element than germanium,” explains Duc Hien Nguyen, doctoral researcher and first author of the study. He focused on processing the solid electrolyte using a slurry-based approach, a technique tailored to industrial roll-to-roll production. In this method, the powdery material is mixed with a solvent and binder to form a spreadable suspension (slurry), which is then processed into thin layers. The slurry must be homogeneous and have the right viscosity to achieve a uniform and efficient layer. “The choice of binder is crucial in ensuring consistently high-quality layers. We tested various binders and found that less polar candidates performed better,” Nguyen reports.
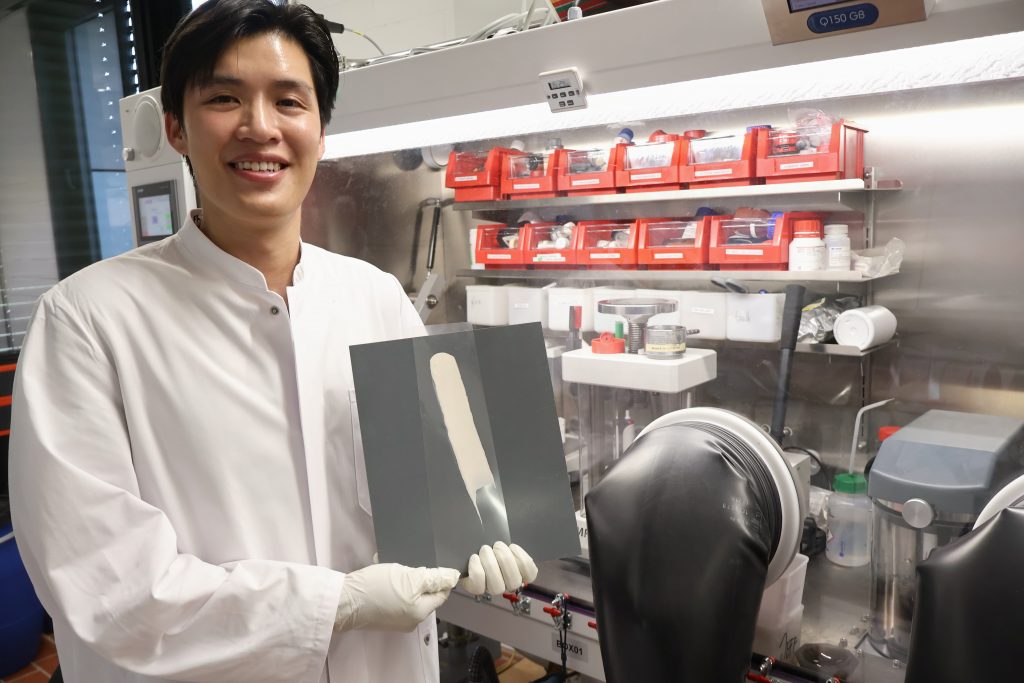
PhD student Duc Hien Nguyen investigated how solid electrolytes can be optimally produced from a slurry that can be brushed. (Photo: Jeff Wijaya/MPG)
Larger Particles, Better Lithium Transport
Nguyen also explored how different particle sizes within the solid electrolyte affect its conductivity. One key finding: the larger the solid particles, the better the ionic conductivity. While particles smaller than 50 µm formed homogeneous and densely packed layers, particles larger than 100 µm yielded significantly better conductivities. “With larger particles, we achieved much higher lithium diffusivities — and that could benefit overall battery performance later on,” Nguyen explains. Although smaller particles offer advantages in processing, they also lead to more grain boundary resistances, which hinder lithium transport. “Our results highlight the delicate balance at play,” says Prof. Lotsch. “Controlling particle size in a targeted way can help improve the microstructure — and thereby the conductivity — of solid electrolytes.”
The study appears in EES Batteries. Funding was provided by the German Research Foundation (DFG) through the e-conversion Excellence Cluster and by FestBatt (Cluster of Competence for Solid-state Batteries) funded by Federal Ministry of Research, Technology and Space (BMFRT).
Publication:
Effect of particle size on the slurry-based processability and conductivity of t-Li7SiPS8;
Duc Hien Nguyen, Lars Grunenberg, Igor Moudrakovski, Kathrin Küster, Bettina V. Lotsch
https://doi.org/10.1039/D5EB00005J
Contact:
Prof. Bettina V. Lotsch
Nanochemistry Department
Max Planck Institute for Solid State Research
b.lotsch@fkf.mpg.de
